In recent days, many people have been confused after seeing headlines claiming that “Anvisa bans fiberglass nails.”
But did Anvisa really ban this nail technique? The answer is no.
In fact, the agency banned two chemical substances that some gel nail products — and, in some cases, fiberglass nail extensions — contained.
In this post, you’ll learn what this decision actually means and review other key regulatory updates in Brazil and abroad that directly affect the medical device and cosmetics industries.
Anvisa bans fiberglass nails? Here’s what was actually banned
Anvisa did not ban fiberglass nails, but instead prohibited the use of TPO (Diphenyl [2,4,6-trimethylbenzoyl] phosphine oxide) and DMPT (N,N-dimethyl-p-toluidine), based on evidence of reproductive toxicity and potential carcinogenicity.
These substances were used in some gel nail products, leading to confusion across social media.
Under the new rule, companies must immediately stop manufacturing and importing these substances.
They must also remove existing stocks from the market within 90 days. After that period, the authority will cancel all product registrations.
In short, Anvisa did not ban the fiberglass nail technique. However, products used in this process must not contain the banned ingredients.
Companies and professionals in the sector should review labels, suppliers, and registrations to ensure full compliance with the new regulation.
Saline solutions now classified as medical devices
Now that it’s clear that Anvisa bans fiberglass nails is misleading, another key update comes from Anvisa’s reclassification of saline solutions, which were previously regulated as medicines.
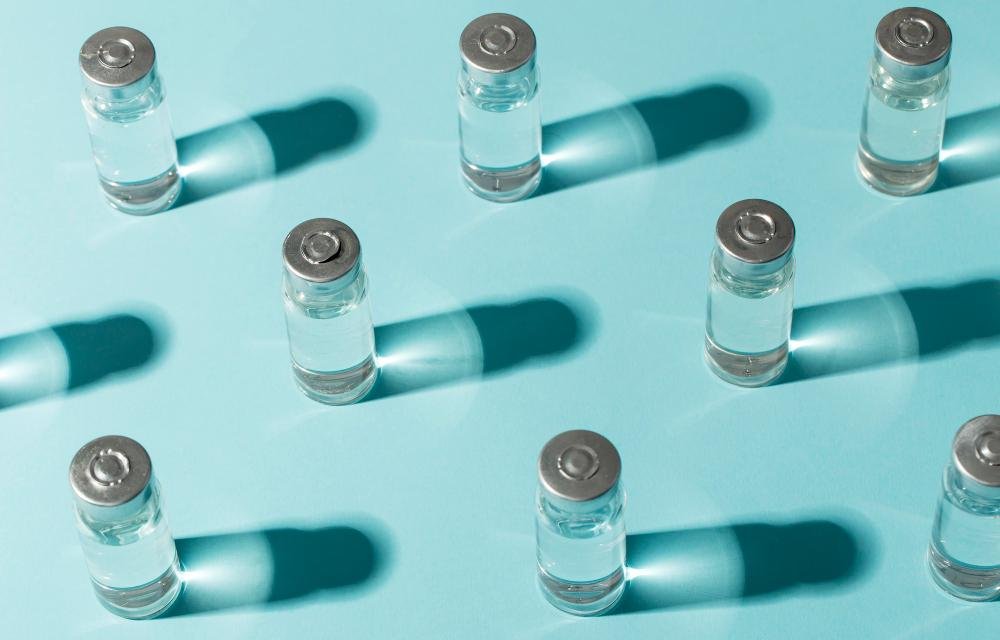
The new regulation classifies saline and irrigation solutions as medical devices. Companies have until May 30, 2026, to update their registrations and compliance processes.
Global cosmetovigilance market projected to reach USD 18.35 billion by 2034
A new market report indicates that the global cosmetovigilance sector—the monitoring of cosmetic product safety after market release—is experiencing steady growth.
Analysts expect the global market value to rise from USD 12.26 billion in 2025 to USD 18.35 billion by 2034, with an average annual growth rate (CAGR) of 4.59%.
This trend reflects stronger regulatory requirements and growing awareness of consumer safety. As a result, cosmetovigilance is becoming a strategic area for brands and cosmetic manufacturers worldwide.
China expands UDI system for medical devices
China’s National Medical Products Administration (NMPA) has released a draft to expand the Unique Device Identification (UDI) system, adding new product categories and strengthening traceability throughout the supply chain.
This move highlights China’s commitment to international standards. The country is aligning its rules with those adopted by the United States and the European Union.
For companies exporting or manufacturing medical devices for the Chinese market, it’s crucial to stay informed and prepare for these upcoming changes.
Why these updates matter
Together, these regulatory developments signal a global shift toward greater transparency and safety, both in cosmetics and in medical devices.
The misconception that “Anvisa bans fiberglass nails” shows how essential clear and technical communication is — regulation exists to protect consumers, not to restrict safe practices.
Companies operating in regulated markets must stay alert to these changes, ensuring their products remain compliant and ready for an increasingly demanding environment.
If you want to stay up to date with news like this, subscribe to the Sobel News Roundup!
It’s free, and you’ll receive the latest regulatory updates that impact your business directly in your inbox.




